Regulatory Consulting Services
Expert guidance for medical devices, pharmaceuticals, food, and cosmetics to navigate US market regulations.
Medical Device Industry
The medical device industry in the U.S. is regulated by the FDA, requiring compliance with various standards including device registration, 510(k) premarket notification, unique device identification, electronic reporting of adverse events, and certification for foreign export.
Pharmaceutical Industry
In the pharmaceutical industry, products are regulated by the FDA based on their intended use, requiring compliance with various standards including drug registration, listing, labeling, and reporting, with additional regulations for products that may fall under both drug and cosmetic categories.
The cosmetic industry is regulated by laws like the FD&C Act, which defines cosmetics by their intended use and does not require FDA approval for most products except for color additives, though compliance with labeling and classification regulations is crucial, and the Modernization of Cosmetics Regulation Act of 2022 introduces new safety requirements.
Cosmetics Industry




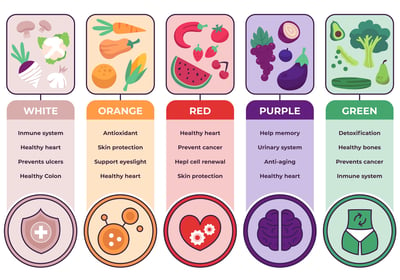
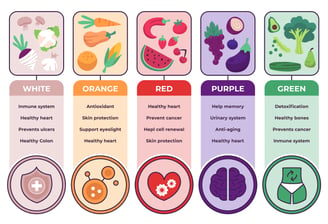
Food Industry
The US FDA regulates most foods and ingredients in interstate commerce, requiring labeling for prepared foods, facility registration for food safety compliance, and provides certificates of registration, while certain exceptions like meat and poultry fall under the USDA's jurisdiction.


CE Marking
RegR Consultants Inc. offers comprehensive CE marking services, guiding businesses through the certification process to ensure their medical devices comply with EU health, safety, and environmental regulations, allowing them to market their products in Europe.
Regr Consultants made our FDA approval process seamless and cost-effective. Highly recommend their expertise!
John Doe


★★★★★
Consulting
Expert guidance for your Regulatory Requirements
Email Us:
© 2024. All rights reserved.